Below is an abbreviated list of the roles microbes play in our lives:
 When
considering the impact of bacteria and other microbes on the earth it is
important to grasp the concept of "MICROENVIRONMENTS"
or "MICROHABITATS". Microenvironments
are small spaces or locations within the Earth's ecosystem where some environmental
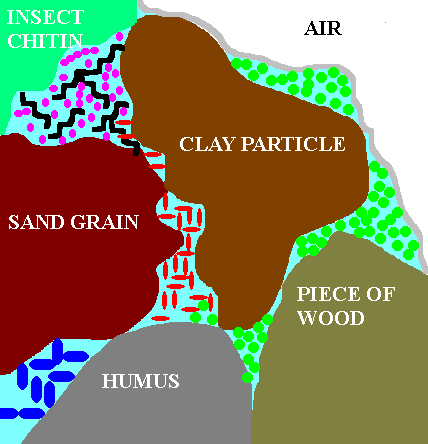
factors differs from another that is nearby. This cartoon illustrates a
microenvironment situation in the soil. For example, within a square cm
of normal soil 100s or even 1000s of different environments (microhabitats)
may exist separated by only a mm or less. One part of the soil may contain
a tiny fragment of organic matter (a cellulose chunk) that is only utilized
(eaten) by a few species of microbes (those that produce cellulase). Thus
mostly cellulase-producing microbes would be found there and they would,
in turn, produce waste products that would change the pH in a sphere ~0.1
mm around the cellulose chunk. Whereas 1 mm away is a different nutrient
source with its unique microbes affecting the immediate environment around
it (e.g. by producing an antibiotic that inhibits likely competitors).
Essentially the entire world is composed of microenvironments. In fact
microenvironments are so prevalent that they are assumed to be far more
common than macroenvironments (like beer fermentation tanks). Even the
water in a lake or the ocean is composed of distinct microenvironments
depending on environmental factors like light, nutrients, temperature etc.
Our mouths contain a host of microhabitats that determine it health and
halitosis.
When
considering the impact of bacteria and other microbes on the earth it is
important to grasp the concept of "MICROENVIRONMENTS"
or "MICROHABITATS". Microenvironments
are small spaces or locations within the Earth's ecosystem where some environmental
factors differs from another that is nearby. This cartoon illustrates a
microenvironment situation in the soil. For example, within a square cm
of normal soil 100s or even 1000s of different environments (microhabitats)
may exist separated by only a mm or less. One part of the soil may contain
a tiny fragment of organic matter (a cellulose chunk) that is only utilized
(eaten) by a few species of microbes (those that produce cellulase). Thus
mostly cellulase-producing microbes would be found there and they would,
in turn, produce waste products that would change the pH in a sphere ~0.1
mm around the cellulose chunk. Whereas 1 mm away is a different nutrient
source with its unique microbes affecting the immediate environment around
it (e.g. by producing an antibiotic that inhibits likely competitors).
Essentially the entire world is composed of microenvironments. In fact
microenvironments are so prevalent that they are assumed to be far more
common than macroenvironments (like beer fermentation tanks). Even the
water in a lake or the ocean is composed of distinct microenvironments
depending on environmental factors like light, nutrients, temperature etc.
Our mouths contain a host of microhabitats that determine it health and
halitosis.
|
|
|
|
One common accommodation is for two species to work together for the mutual benefit of each. Such cases are known as MUTUALISTIC symbioses. In another common symbiotic relationship one of the associates benefits from the association while the other either gains nothing, is harmed or even killed. This form of symbiosis is called PARASITIC. The partner that benefits is called the PATHOGEN or PARASITE, while the other member is called the HOST. Symbioses can be simple, involving only two components or extremely complex, involving many participants interacting in very intricate and complex ways. The degree of interdependence can vary from organisms that will easily grow in the absence of other members of a symbiotic relationship, to situations where none of the organisms involved can live normal lives without the other symbionts. The latter case is complicated by situations where symbionts can be grown independently in the laboratory, but are not found in natural environments living apart.
Symbioses are so common that some scientists argue that
all living organisms on earth exists in some symbiosis with another. Take
for example, that most common gut bacterium E. coli that
inhabits the intestine of many animals, including ourselves. We know that
it is quite capable of living independently outside of the gut because
we use it as an indicator of #fecal pollution in our drinking #water and
#food. However, its natural habitat or "home" is assumed to be the intestine
of many animals. Another way of considering this is that evolution has
equipped E. coli for surviving best in a gut, all other environments
being secondary. Indeed the dictates of #natural selection require that
every organism be adapted to best fit one particular environmental niche.
As a prideful fellow human you may take umbrage at that statement, citing
the many environments humans (and coyotes, rats and a few other species)
survive well in. However, in the case of humans, we basically cheat as
we simply "take our preferred environment" with us wherever we go; consider
the astronaut and the aquanaut. Basically, the other examples given also
do the same thing in their own engaging way.
|
|
|
|
The activity of microorganisms on the earth is often rather grandly referred to as their "GEOCHEMICAL" activities. All this means is that they do their chemistry on a grand scale and perform a large variety of fascinating chemical processes that amuse, awe and enrich us in many cases. However, they are, quite simply, going about their daily lives, just as we do. That is, we eat, digest, engage in various activities to pass the time, and defecate, all of which affects the environment around us, yet few of us think of ourselves as being "geochemical agents" (have you visited a strip mine lately?). All life transforms chemicals through their biochemical activities. For example, photosynthetic organisms convert carbon dioxide, water and light to organic matter, but somewhere down the line that chemistry is reversed and the polymers made in the synthetic process are converted back into the original chemicals (CO2 and H2O) from whence they came. This is where the concept CYCLE comes in (or as it is so poetically stated in the Bible: "dust to dust").
All the nutrients of life endlessly turnover in a cyclic way and each nutrient has a cycle involving a group of microorganisms that are responsible for carrying out this process. A given cycle is often viewed as starting with basic elements being converted into larger, complex organic polymers as discussed in #Chap. 6. Once the cells containing these polymers die, degradation, or MINERALIZATION as the process is often called, occurs and the polymers are converted to the basic chemical precursors of new life. A simplistic way to think of the process is life to dead organic matter to fertilizer to new life forever.
|
|
All living organisms carry out #catabolism which results in degradation and all living organism carry out #anabolic processes which expend energy to construct macromolecules or to move etc. Most of the dead cow you ate in your last hamburger was catabolized and its mineralized component parts expelled from your body through your lungs, urine or feces. To make it easier to understand I will illustrate several cycles of nutrient elements.
The Carbon Cycle: This is an easy one. Carbon dioxide + water + energy + plant/microbes = organic molecules in live organisms = organic molecules in dead organisms + oxygen + microbes = energy + carbon dioxide + water; "round 'n round she goes, where she stops nobody knows". However, some carbon stays around for a really long time in the form of coal, methane, oil and CaCO3 deposits.
One important component of the carbon cycle is methane (CH4) production and metabolism (#oxidation). Methane is a major byproduct of many anaerobic biochemical processes, including those that occur in our very own intestines. Methane is much more effective than carbon dioxide in absorbing and radiating energy (HEAT) back to the earth; i.e., methane is a major greenhouse gas. Methane-producing microbes produce about 400 million metric tons of methane each year, but only under anaerobic conditions (inside rumens, insects, wetlands, rice paddies, sewage digesters, landfills, biogas generators (Sci.278:1413[1997]). The increase in the greenhouse gas methane that is attributed to man is ~1%/year. Conversely, methane is an important carbon and energy source for those microbes that are able to metabolize (oxidize) it into carbon dioxide and water. That is, methane is a major component of the carbon-cycle.
The Nitrogen Cycle: Although nitrogen gas (N2) constitutes 80% of the earth's atmosphere, N2 can not be used by most forms of life. However, nitrogen is a major component of protein and nucleic acids and so is a major #required nutrient. The majority of organisms can only use nitrogen if it is in the form of ammonia, nitrates or nitrites. But these are scarce chemicals in nature and nitrogen deficiencies often limit crop yields. Fortunately, there are a number of microbes that are able to convert the N2 in the air to ammonium nitrogen. These organisms, (and this will surprise you) are called NITROGEN-FIXING bacteria. The only other natural source of usable nitrogen for plants is from lightening.
The nitrogen cycle starts with N2-fixing bacteria. The nitrogen-fixing bacteria are able to take N2 gas + a lot of energy + a lot of electrons and convert it to ammonia (NH3) which they use to make the many nitrogen-containing organic molecules they required to grow and make offspring. When they, and any other living organism die, much of their organic-bound nitrogen is released by microbes decomposing the dead as ammonia (some of it, however is used directly by the degrading organisms); which you can smell when you turn over a compost pile. The ammonia is oxidized to nitrite (NO2) by a group of specialized microorganisms to extract the energy trapped in it. Another group of bacteria subsequently oxidize the nitrite to nitrate (NO3) and squeeze out some more energy. Ammonia, nitrite and nitrate can be assimilated by plants and a lot of microbes and converted into nitrogen-containing organic molecules.
The final chapter to the nitrogen cycle occurs under anaerobic conditions where still another group of bacteria use nitrite/nitrate as electron acceptors (substituting for oxygen) and in the process reduce the nitrogen back into the atmosphere as N2 gas, thus completing the cycle. In summary nitrogen is continuously cycled from N2 gas into living organisms and back into N2 gas.
FAQ: "So, why should we worry about the N-cycle, why should it interest a non-scientist?"
Answer: Because it effects the price of everything you eat that's why. Since the growth of plants is limited by the small quantity of natural nitrogen in the soil farmers must add nitrogen to the soil in huge quantities at an equivalent huge expense (which they pass on to you when you buy a hamburger etc.) in order to maximize the yield of their crops. Nitrogen fertilizer (mostly ammonia) is expensive to make and gobbles up oodles of fuel (gas/oil etc.) which is included in your pizza and beer bill. Yet lots of microbes turn N2 into ammonia more or less for free so if we could find some way to tap into that systemů..well, it would make the California gold rush look like winning a two-dollar lottery. As you will see in the next section, we are able to use N-fixing microbes to some extent to our benefit.
Other Nutrient Cycles:
There are two other cycles that are often presented in this section. They
are the sulfur and phosphorous
cycles. However, I think you are all clever enough to realize by now that
all you do is substitute the terms "sulfur" or "phosphorous" for "carbon"
or "nitrogen" in the two examples given above and you'll get the general
picture. Real microbiologists have to learn all the names of the various
unique bacteria that perform the chemical steps, as well as the chemical
formulas of each step.
In what can be considered a classical case of coevolution, the ruminants have evolved a digestive tract that is an optimum environment for the rumen microbes, while the microbes have simultaneously evolved so as to live optimally in the environment found in the ruminant digestive system. Neither the rumen microbes or the ruminants are able to exist naturally without the other; that is they are CODEPENDENT upon each for life. An example illustrating this is the problem that forest game managers encountered when they first attempted to transfer ruminants, like deer and elk, from one forest to another. In many of these early relocation attempts the transferred animals died from starvation even though ample grass was available at the new site. The puzzle was compounded because the area usually already contained the same species as the transported ruminants that were living without problems on the same diet. It turned out that the grasses in the two areas differed in composition enough so that the rumen microbes in the transported animals were so well adapted to the grasses in the animals' original location that they were unable to digest the grasses in the new place efficiently. The answer was to slowly introduce those animals to be moved, to grasses brought from the new area over a period of time. This allowed the development of rumen microbes population that was capable of utilizing the new diet.
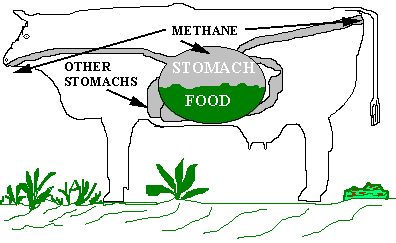 The
digestive tract of ruminants contain four successive stomachs, the first
two of which are essentially culture containers for the growth of a variety
of microbes, including bacteria, fungi and protozoa. The cow acts mainly
as a mechanical grinding machine that collects the grass and chews it up
into small fragments that provide a large surface area which is more easily
digested by the rumen microbes. As the grass is chewed it is mixed with
salvia that buffers (maintains) the pH at an #optimal levels. The
rumen microbes secrete enzymes that breakdown the cellulose and other complex
polysaccharides into small sugar units that are subsequently fermented
by the microbes. Because the environment in the digestive tract is anaerobic
the sugars are fermented to acetic, propionic and butyric acids and to
carbon dioxide and methane. The fatty acids are absorbed by the cow as
nutrients and the gases are released by frequent belching and anal venting.
The
digestive tract of ruminants contain four successive stomachs, the first
two of which are essentially culture containers for the growth of a variety
of microbes, including bacteria, fungi and protozoa. The cow acts mainly
as a mechanical grinding machine that collects the grass and chews it up
into small fragments that provide a large surface area which is more easily
digested by the rumen microbes. As the grass is chewed it is mixed with
salvia that buffers (maintains) the pH at an #optimal levels. The
rumen microbes secrete enzymes that breakdown the cellulose and other complex
polysaccharides into small sugar units that are subsequently fermented
by the microbes. Because the environment in the digestive tract is anaerobic
the sugars are fermented to acetic, propionic and butyric acids and to
carbon dioxide and methane. The fatty acids are absorbed by the cow as
nutrients and the gases are released by frequent belching and anal venting.
The microbes and the undigested food is passed into the lower two stomachs which produce #proteases. These enzyme digest the microbes, releasing their amino acids which are, in turn, absorbed by the cow as a source of nitrogenous nutrient. Other nutrients, such as vitamins and fats are also obtained from the digested microbes. Since the 60 to 80 liters of gas (CO2 and CH4) that are released per day per adult cow are both "greenhouse" gasses, cattle, and other commercial ruminants, are considered by some to make a major contribution to global warming.
One could ask a chicken/egg type question here which is: Were cattle primarily designed to provide a suitable environment for a group of microbes or is it the other way around? What do you think?
People who studied these restorative plants noticed that they contained small tumor-like growths, called #NODULES, on their roots. A close examination of these structures showed that they had a pink color. Eventually, science developed to the point where chemical analysis showed that one of the chemicals that was absent or in low quantities in unproductive soils was nitrogen. Further, it was then discovered that following the rotation with a restorative plant, now called LEGUMINOUS PLANTS, the level of nitrogen was increased significantly. From these and other studies it was determined that one of the common nutrients often limiting maximal crop yield was nitrogen and that the leguminous plants put nitrogen into the soil. How the latter was done required the development of the science of microbiology.
The wide spread usage of crop rotation of legumes like alfalfa in the 1700 and 1800s contributed to a population explosion in Western Europe at a time when workers were needed for the developing Industrial Revolution. Naturally, the importance of crop rotation in the general economy attracted scientific investigation in the hopes of making it even more efficient. Studies showed that legumes converted nitrogen gas to organic nitrogen in a process that came to be called NITROGEN FIXATION. Further, if legumes lacked the nodules, they did not fix nitrogen. Microscopic examination of the nodules showed them to be filled with small bodies of the size associated with bacteria. Subsequently, using the technique pioneered by #R. Koch, bacteria were isolated from these nodules. It was also found that leguminous plants grown from seeds treated to kill any associated bacteria neither fixed nitrogen or produced nodules when cultivated in sterile soil. However, when the bacteria, the Rhizobia, isolated from the nodules were inoculated onto the non-nitrogen-fixing plants, they developed nodules and began fixing nitrogen in the usual fashion.
Subsequently microbiologists have found that other bacteria are able to fix nitrogen, some by themselves and others in symbiotic relationships with specific nonlegumenous plants. However, the legumes/Rhizobia relationship remains the most important commercially nitrogen-fixing symbiosis. The relationship is very complex and far from being completely understood. For example, the Rhizobia show significant specificity regarding the species of legume with which they will form a symbiotic relationship. Thus, while it is now routine that the seeds of commercial leguminous plants are coated with Rhizobia, the strain of Rhizobia used must match the particular legume or the system will not work.
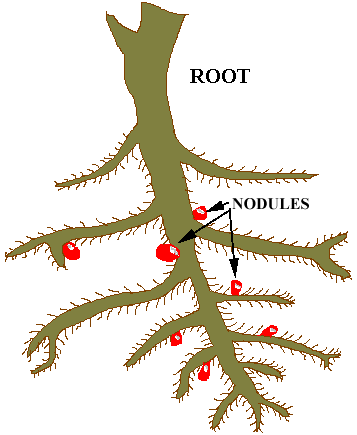 The
life cycle of the Rhizobia has been intensely studied. It is now known
that a particular strain of Rhizobium in the soil is attracted to its particular
host. Since the Rhizobia are motile they actually swim to their host. Once
they reach the host's root system they attach to a single root hair and
releases substances that stimulate the hair to allow the Rhizobium to penetrate
it. The Rhizobium proceeds to invade certain cells in the root where they
produce chemicals that stimulate the growth of these cells to produce the
tumor-like nodule. Inside the nodule the Rhizobia change their form to
cells called BACTEROIDS. The bacteroids
convert N2 to ammonia using the energy they obtain by metabolizing
nutrients provided them by the plant host. The fixed nitrogen is subsequently
made available to the host plant. When the host plants die their nitrogen
is returned to the soil for use by other organisms. In many cases today
the farmers plow the entire legume into the soil to insure that as much
as possible of the fixed nitrogen is available to subsequent crops.
The
life cycle of the Rhizobia has been intensely studied. It is now known
that a particular strain of Rhizobium in the soil is attracted to its particular
host. Since the Rhizobia are motile they actually swim to their host. Once
they reach the host's root system they attach to a single root hair and
releases substances that stimulate the hair to allow the Rhizobium to penetrate
it. The Rhizobium proceeds to invade certain cells in the root where they
produce chemicals that stimulate the growth of these cells to produce the
tumor-like nodule. Inside the nodule the Rhizobia change their form to
cells called BACTEROIDS. The bacteroids
convert N2 to ammonia using the energy they obtain by metabolizing
nutrients provided them by the plant host. The fixed nitrogen is subsequently
made available to the host plant. When the host plants die their nitrogen
is returned to the soil for use by other organisms. In many cases today
the farmers plow the entire legume into the soil to insure that as much
as possible of the fixed nitrogen is available to subsequent crops.
Crop rotation is illustrated in the Palouse here by the use of pea plants (a type of legume) used by the wheat farmers to revitalize fields depleted of nitrogen by the intense cultivation of wheat.
In many ways mutualistic symbiotic relationships are like
employee/employer relationships. Both of the participants contribute something
that the other requires to survive. Without workers a business can't produce
goods, but without employment workers can not purchase the necessities
of life.
Copyright © Dr. R. E. Hurlbert, 1998.
This material may be used for educational purposes only and may not
be duplicated for commercial purposes.
E-MAIL: hurlbert@wsu.edu or hurlbert@pullman.com